AIM
The aim of this study was to investigate the feasibility of and compare the comfort and preference of self-collection combined nose and throat (CTN), saliva and Rhinoswab, a novel anterior nasal swab (ANS) for detection of SARS CoV-2 in children 4-18 years of age.
METHOD
The study was conducted by the Murdoch Children’s Research Institute (MCRI) at the Royal Children’s Hospital Melbourne (RCH), between 15 February and May 2022 with 53 children between the ages of 4-18 years, of which 43 were confirmed COVID-19 positive in the prior 7 days and 9 were household contacts of these confirmed cases.
Self-collected CTN, ANS (Rhinoswab Junior) and saliva swabs were obtained concurrently. Swabs were collected by the patient or assisted by the parent, without clinician assistance. Swabs were collected at home, Emergency Department, or inpatient ward.
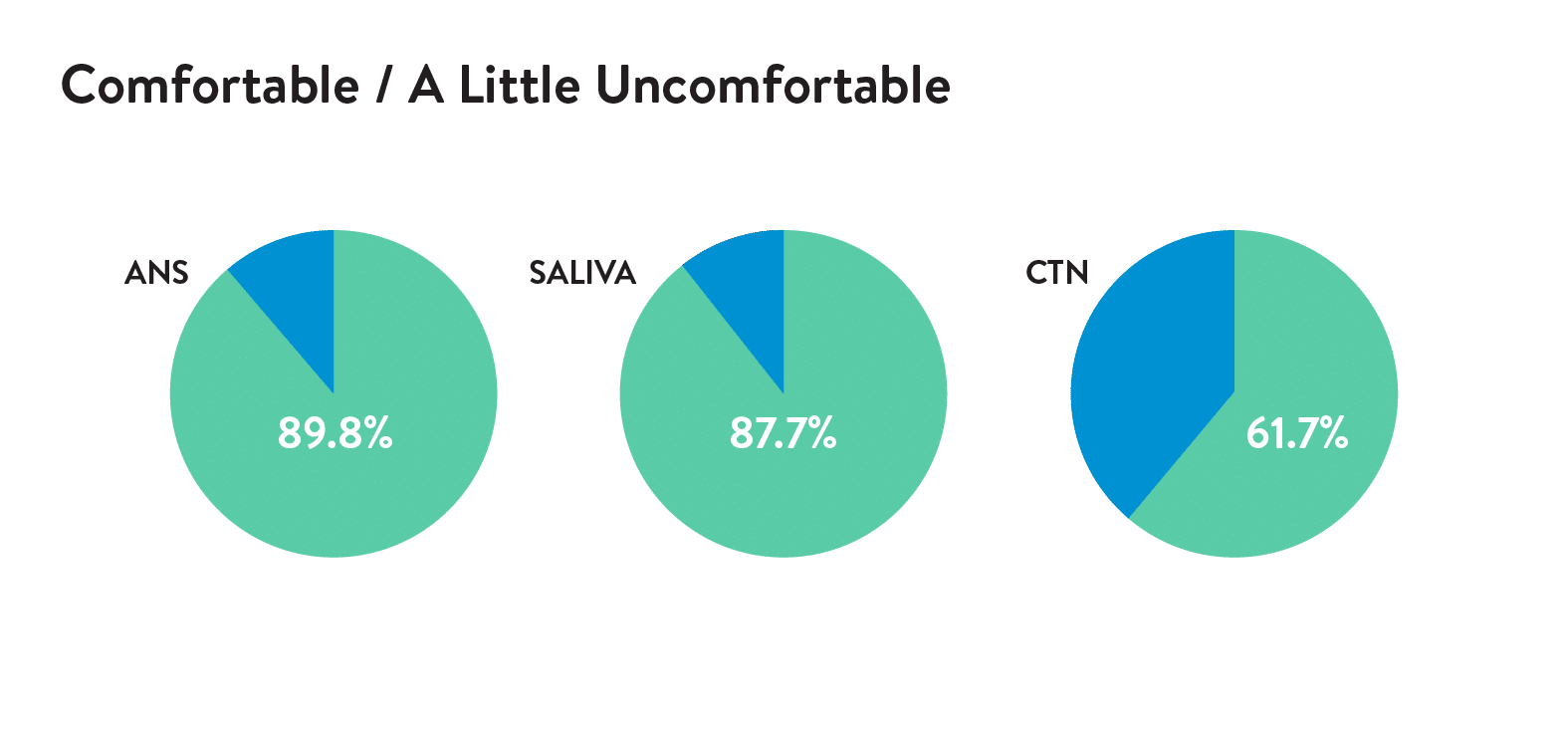
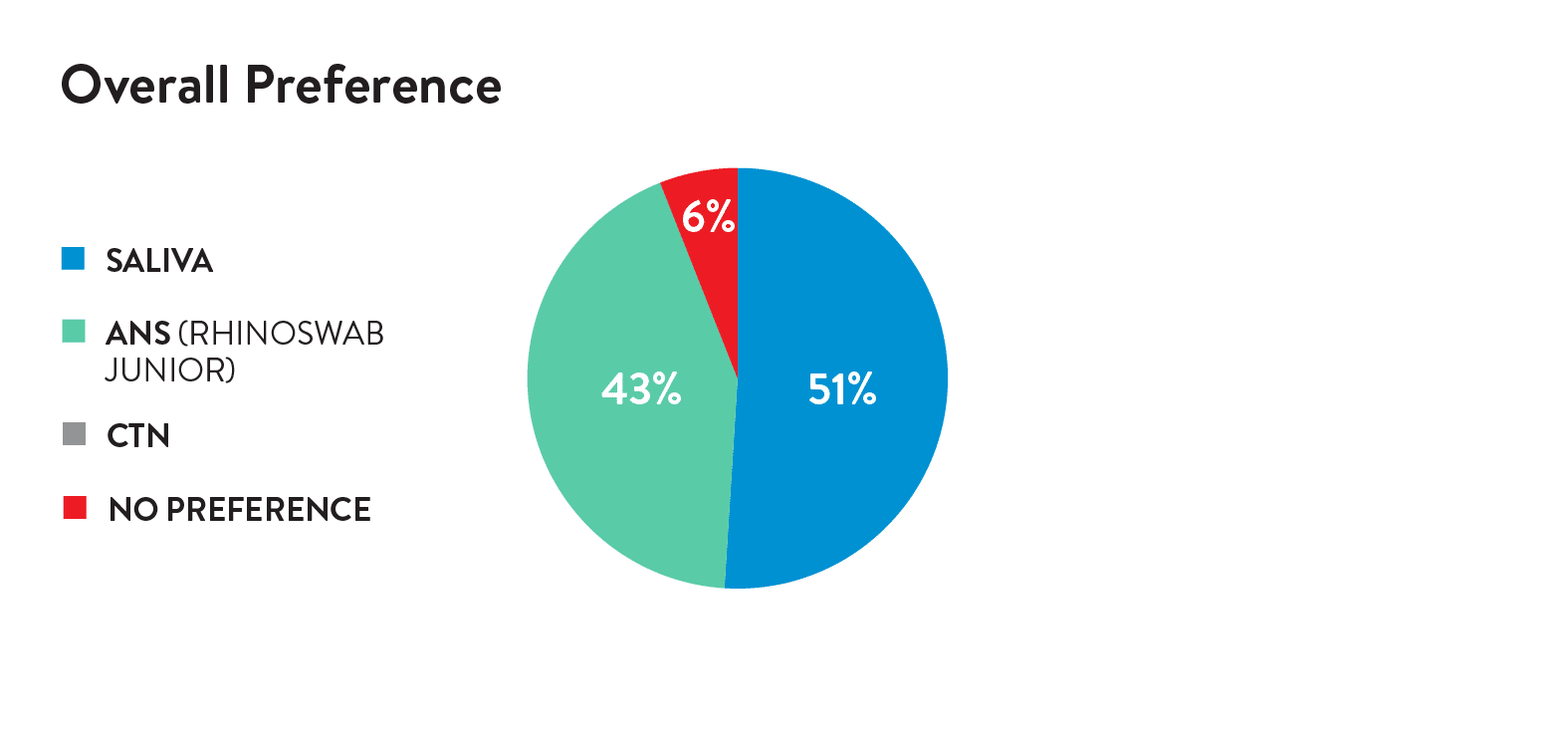
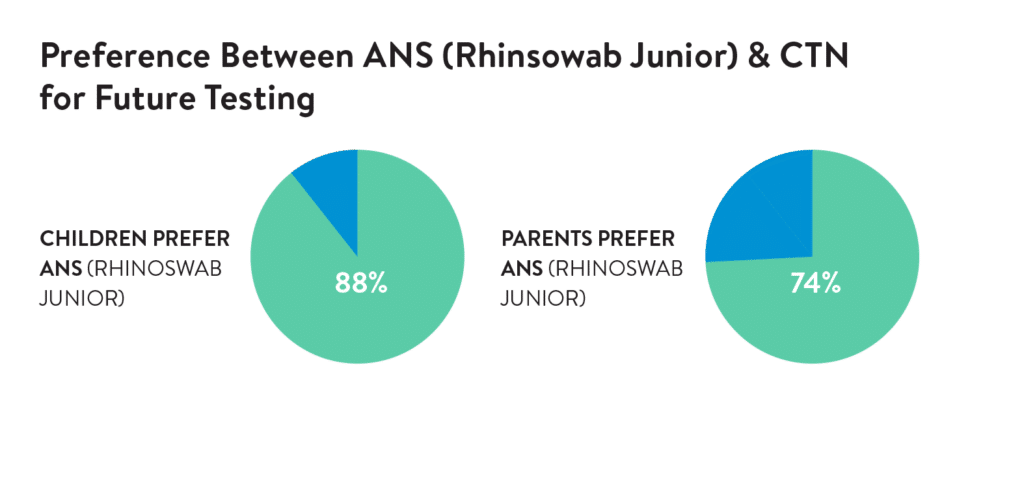
Acceptability was assessed by an electronic survey following the swabs. A 5-point Likert scale or Wong-Baker FACES scale were used to rate comfort and preference by the child (self-report) or parent.
RESULTS
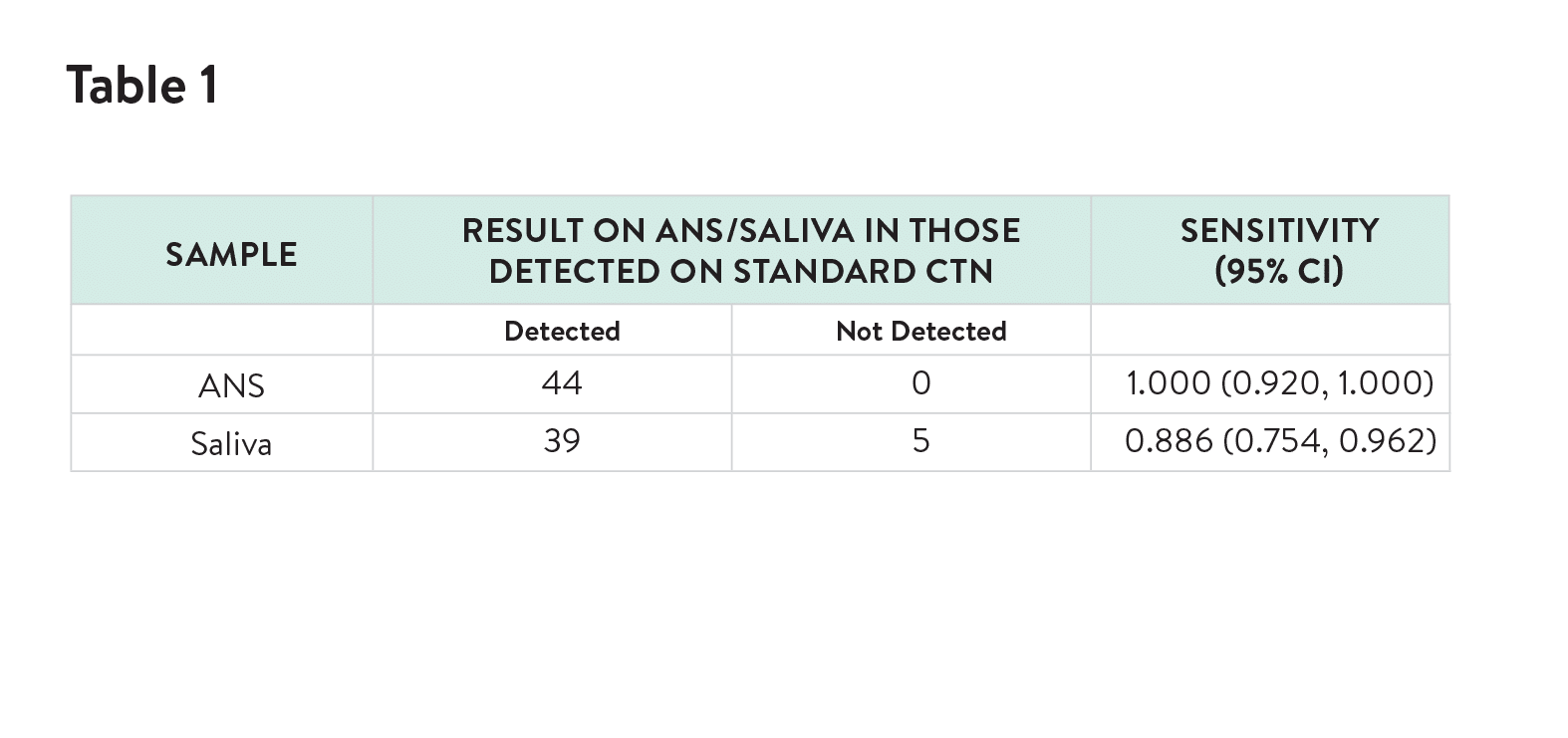
The novel ANS (Rhinoswab Junior) was found to provide a more sensitive method for detection of SARS-CoV-2 in children than saliva (Table 1) with similar comfort scores by children and parents.