BACKGROUND
Detection of respiratory viruses requires the collection of samples for testing, however, nasal and throat swabs can cause discomfort and procedural anxiety in children. Respiratory sampling methods which are accurate and less invasive are needed.
AIM
To determine whether Rhinoswab Junior (ANS) is a suitable alternative to the standard combined nose and throat (CTN) swOutcomes included:
- To determine the sensitivity and specificity of Rhinoswab Junior versus a standard swab (CTN)
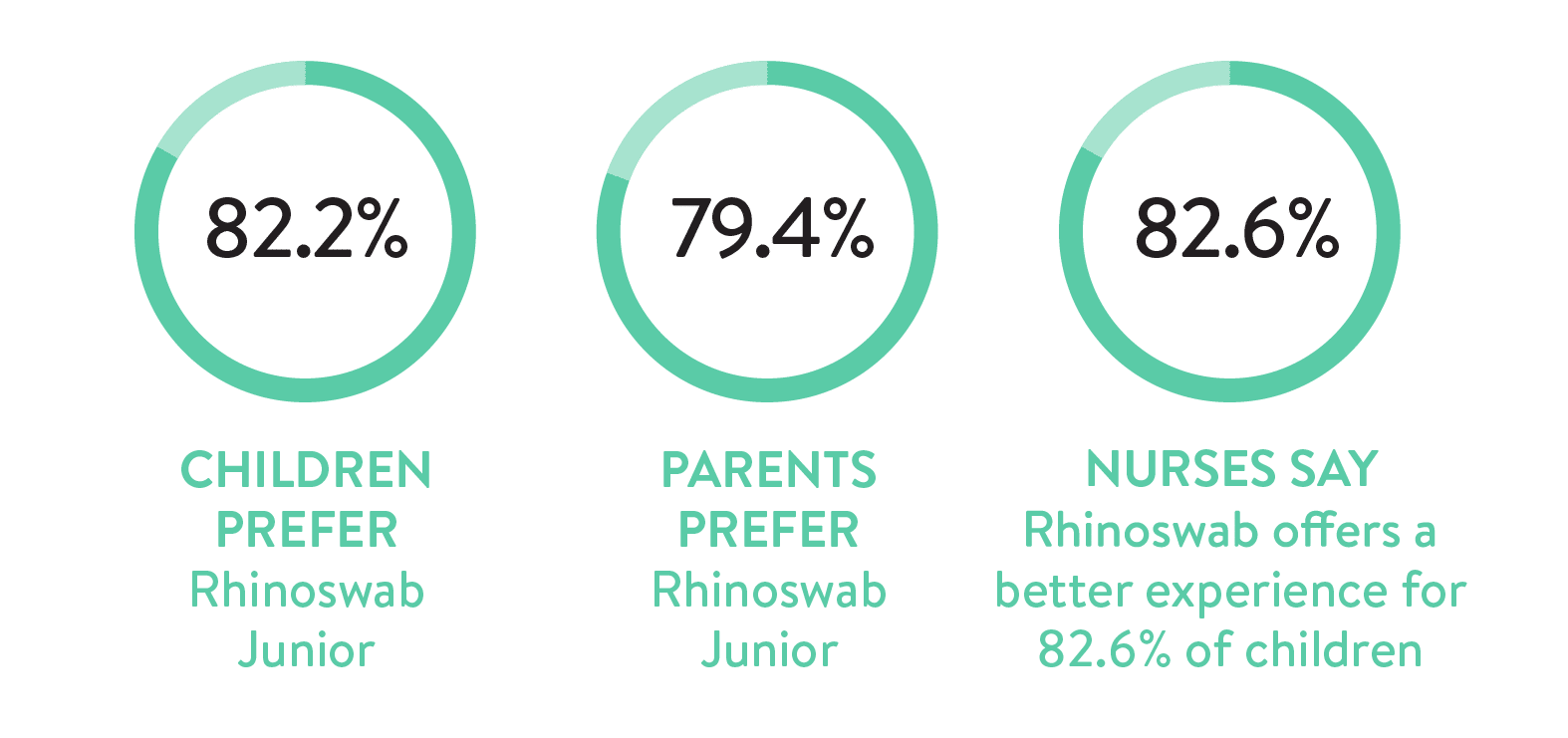
- To measure the comfort and preference of the Rhinoswab versus a standard swab (CTN) for detection of respiratory viruses in symptomatic children.
METHOD
Rhinoswab Junior was tested side by side with the standard combined throat and nose swab in 254 children aged 4-18 years at Murdoch Children’s Research Institute (Melbourne, 2022).
Rhinoswab was inserted for 60 seconds, followed by 15 seconds of “wiggle” time inside the nose. The combined nose and throat swab was completed by the study nurse using a flocked swab (Copan Diagnostics Inc, Corona, CA).
A user experience survey was administered to the children, their guardians, and the test nurses to measure feedback on factors such as comfort and swab preference. The respiratory panel was made up of 12 viruses including SARS-CoV-2.
RESULTS
The study concluded that Rhinoswab Junior had high sensitivity (91%) and specificity (99%) in the detection of respiratory viruses, which was comparable to the current CTN standard of care. In addition, it provided a more comfortable experience and was preferred by children, parents/guardians and nurses.