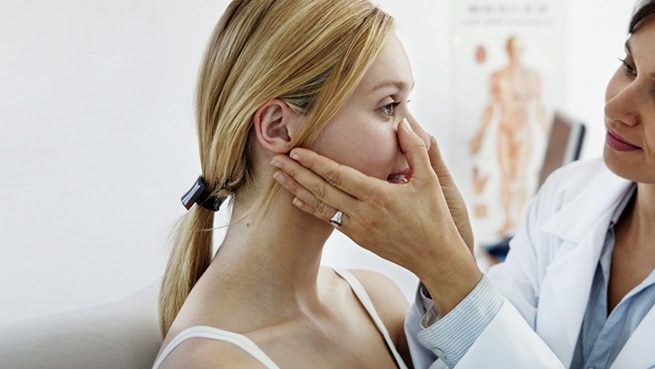
Wearable nasal technology
Rhinomed is a global medical device company enhancing respiration, sleep, diagnosis of upper respiratory disease and nasal drug delivery.
Introducing RHINOSWAB
A more comfortable nasal swab for COVID-19 and upper respiratory pathogens including influenza and RSV.


And RHINOSWAB JUNIOR
The happy way to swab children.
Rhinomed
knows noses
Rhinomed is a wearable nasal technology company that seeks to radically improve the way you breathe, sleep, maintain your health, diagnose disease and take medication.
We create medical devices that are used by millions of people globally.
With a pipeline of compelling innovation focused on clear unmet clinical needs, we invite you to experience firsthand the difference Rhinomed can make to your way of life.
News
- Rhinomed launches fully underwritten rights issueRhinomed today announces a fully underwritten non-renounceable rights issue of fully paid ordinary shares, to raise approximately A$3.54 million. The funds raised will be used to drive growth in Rhinomed’s … Read more
- European Launch of Revolutionary New Swab for Testing Children for Respiratory DiseasesThe world’s most child-friendly respiratory swab was launched in Europe today at Medica 2022 in Dusseldorf, Germany. Rhinoswab Junior is the first nasal swab developed specifically for children. Its unique design … Read more
- New study says there is a better swab for testing children for covid and other virusesA new study suggests the traditional swab suffers from strong reluctance for use for children and a new Melbourne-designed Rhinoswab Junior is the preferred method of sample collection. The reluctance … Read more
- Study confirms new nasal swab is most accurate, comfortable and easy for kidsRhinomed is pleased to advise of the forthcoming publication of a further study and data by researchers from the Murdoch Children’s Research Institute and the Royal Children’s Hospital (RCH) Melbourne confirming the … Read more
- Surescreen Australia & Rhinomed – Rhinoswab Supply DealRhinomed Limited is pleased to report that it has finalised an exclusive supply agreement with SureScreen Australia to supply Rhinoswabs and Rhinoswab Juniors for inclusion in SureScreen’s class-leading range of … Read more
- Victorian Government orders RhinoswabRhinomed has received an initial purchase order for one million Rhinoswabs from the Victorian Department of Health (DH) to support Victoria’s testing capability. This follows the recent NSW Health Pathology … Read more