THE RELIABLE & HAPPY WAY TO SWAB CHILDREN FOR RESPIRATORY VIRUSES
Rhinoswab Junior has been shown in clinical trials to be equivalent to the combined nose and throat swab in efficacy, but preferred by children and their parents for future tests*.
Introducing rhinoswab Junior

Designed for children
Clinically equivalent to combined nose and throat swab
Standardized sampling process for improved reliability
Validated for PCR and rapid antigen tests
Preferred by children and parents for future tests
Rated by nurses for superior user experience in children
Self collection and novelty features reduce anxiety over testing
Rhinoswab Junior is listed for sale as a Class 1 device in Europe (CE Mark), in the United States with the US Food and Drug Administration (FDA), in the UK with the Medicines and Healthcare products Regulatory Agency (MHRA), in Canada with a Medical Device Establishment Licence (MDEL) and in Australia with the Therapeutic Goods Administration (TGA).
A new standard in sample collection
Standardized and easy sample collection
Rhinoswab Junior’s standardized and comfortable sample collection process empowers children to take their own sample under adult supervision.
Less intrusive, more comfortable & pain free, Rhinoswab Junior reduces anxiety in children and their parents over testing in both clinical and at home settings.
Rhinoswab Junior’s child friendly features add distraction and fun to the testing process.

Rhinsowab Junior is compatible with both laboratory based RT-PCR testing and rapid antigen testing
Rhinoswab Junior Clinical Trials
Less Invasive SARS-COV-2 testing for children:
A comparison of saliva and a novel anterior nasal swab
Tosif, S., Lee ,L., Nguyen, J., Selman, C., Grobler, A.C., McMinn, A., Steer, A., Babl F., Daley A., Crawford, N. 2022. Less invasive SARS-CoV-2 testing for children: A comparison of saliva and a novel Anterior Nasal Swab Medrxiv.org Pre-Print
AIM
The aim of this study was to investigate the feasibility of and compare the comfort and preference of self-collection combined nose and throat (CTN), saliva and Rhinoswab, a novel anterior nasal swab (ANS) for detection of SARS CoV-2 in children 4-18 years of age.
METHOD
The study was conducted by the Murdoch Children’s Research Institute (MCRI) at the Royal Children’s Hospital Melbourne (RCH), between 15 February and May 2022 with 53 children between the ages of 4-18 years, of which 43 were confirmed COVID-19 positive in the prior 7 days and 9 were household contacts of these confirmed cases.
Self-collected CTN, ANS (Rhinoswab Junior) and saliva swabs were obtained concurrently. Swabs were collected by the patient or assisted by the parent, without clinician assistance. Swabs were collected at home, Emergency Department, or inpatient ward.
Acceptability was assessed by an electronic survey following the swabs. A 5-point Likert scale or Wong-Baker FACES scale were used to rate comfort and preference by the child (self-report) or parent.
RESULTS
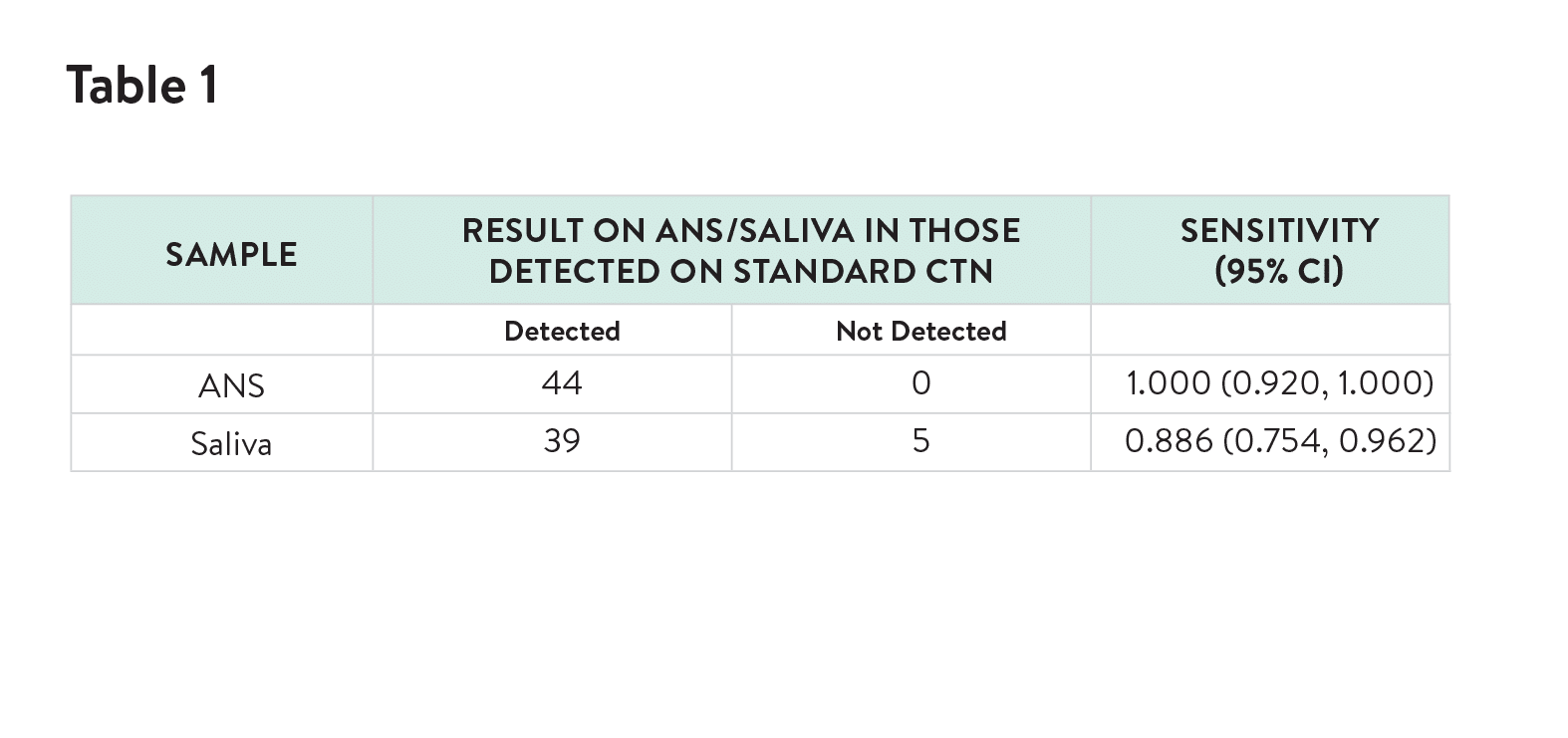
The novel ANS (Rhinoswab Junior) was found to provide a more sensitive method for detection of SARS-CoV-2 in children than saliva (Table 1) with similar comfort scores by children and parents.
Financial sponsor: Rhinomed
A novel anterior nasal swab to detect respiratory viruses:
a prospective study of diagnostic sensitivity and specificity
Tosif, S., Lee ,L., Nguyen, J., Overmars, I., Selman, C., Grobler, A.C., McMinn, A., Waller, G., McNab, S., Jarvis, T., Steer, A., Babl F., Daley A., Crawford, N. A novel anterior nasal swab to detect respiratory viruses: a prospective study of diagnostic sensitivity and specificity. BMC Pediatrics (2023) 23:201
BACKGROUND
Detection of respiratory viruses requires the collection of samples for testing, however, nasal and throat swabs can cause discomfort and procedural anxiety in children. Respiratory sampling methods which are accurate and less invasive are needed.
AIM
To determine whether Rhinoswab Junior (ANS) is a suitable alternative to the standard combined nose and throat (CTN) swOutcomes included:
- To determine the sensitivity and specificity of Rhinoswab Junior versus a standard swab (CTN)
- To measure the comfort and preference of the Rhinoswab versus a standard swab (CTN) for detection of respiratory viruses in symptomatic children.
METHOD
Rhinoswab Junior was tested side by side with the standard combined throat and nose swab in 254 children aged 4-18 years at Murdoch Children’s Research Institute (Melbourne, 2022).
Rhinoswab was inserted for 60 seconds, followed by 15 seconds of “wiggle” time inside the nose. The combined nose and throat swab was completed by the study nurse using a flocked swab (Copan Diagnostics Inc, Corona, CA).
A user experience survey was administered to the children, their guardians, and the test nurses to measure feedback on factors such as comfort and swab preference. The respiratory panel was made up of 12 viruses including SARS-CoV-2.
RESULTS
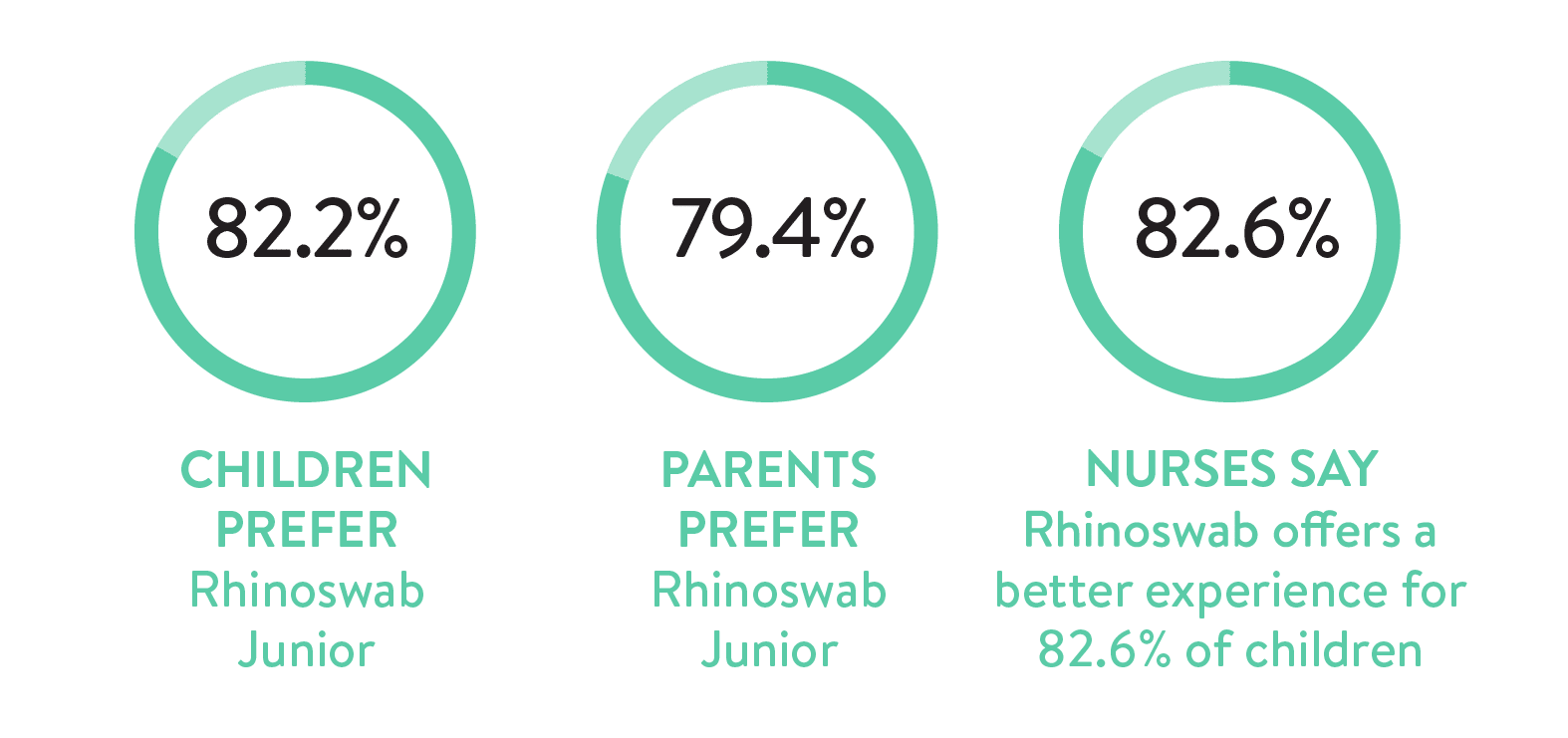
The study concluded that Rhinoswab Junior had high sensitivity (91%) and specificity (99%) in the detection of respiratory viruses, which was comparable to the current CTN standard of care. In addition, it provided a more comfortable experience and was preferred by children, parents/guardians and nurses.
Financial sponsor: Rhinomed



Downloads & Specifications
Please consult our product catalogue to see the full range of offerings from the Rhinoswab and Rhinsowab Junior product ranges. Please choose the correct catalogue based on your location.
FAQs
Yes. Rhinoswab and Rhinsowab Junior can be used with rt-PCR testing.
Rhinoswab is compatible with most existing pathology workflows and standard transport media and collection tubes. In some cases, automated liquid handling equipment require a change of script for the adult swab.
Yes. Rhinoswab is listed for sale as a Class 1 device in Europe (CE Mark), in the United States with the US Food and Drug Administration (FDA), in the UK with the Medicines and Healthcare products Regulatory Agency (MHRA), in Canada with a Medical Device Establishment Licence (MDEL) and in Australia with the Therapeutic Goods Administration (TGA).
Rhinoswab may be administered directly by a healthcare professional, or self-administered by adolescents (12 years and older) and adults.
Users under the age of 16 years, or those challenged by vision, cognitive, dexterity, literacy or language limitations, may require the assistance of an adult aged 21 years and over.
Rhinoswab Junior is designed for children aged 4 years and over.
Both Rhinoswab and Rhinoswab Junior have been validated in clinical trials as comparable to the standard combined throat and nose swab.
No. Rhinoswab and Rhinoswab Junior are self administered.
Rhinoswab is designed to be self administered to minimise risk to health care workers. Health care workers may supervise the sample collection process if necessary for those who are under 16 years of age or those who have cognitive or other challenges.
Yes. A usability study showed that 77.1% of participants found the swab to be very comfortable or comfortable and a further 20.8% found it to be neither comfortable nor uncomfortable.
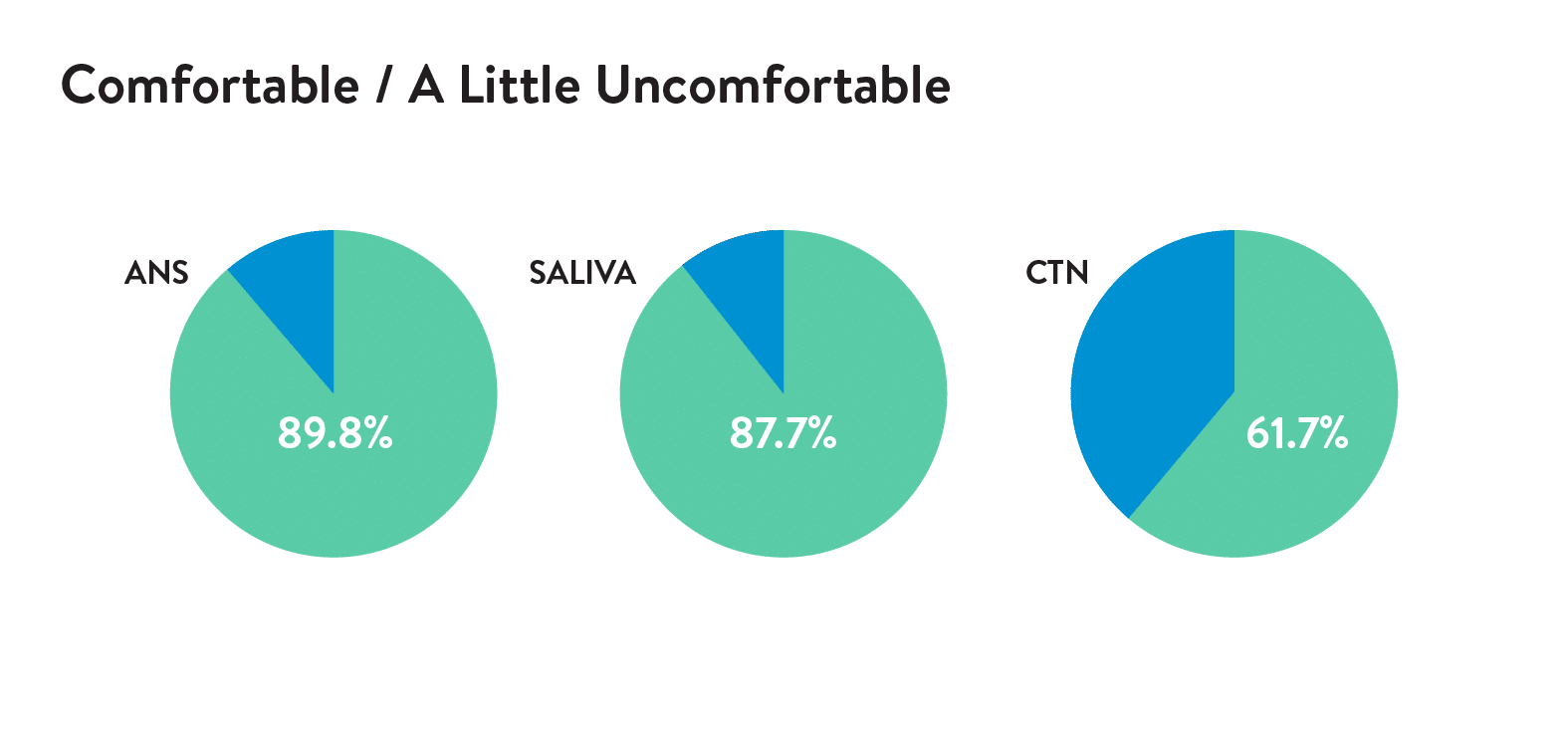
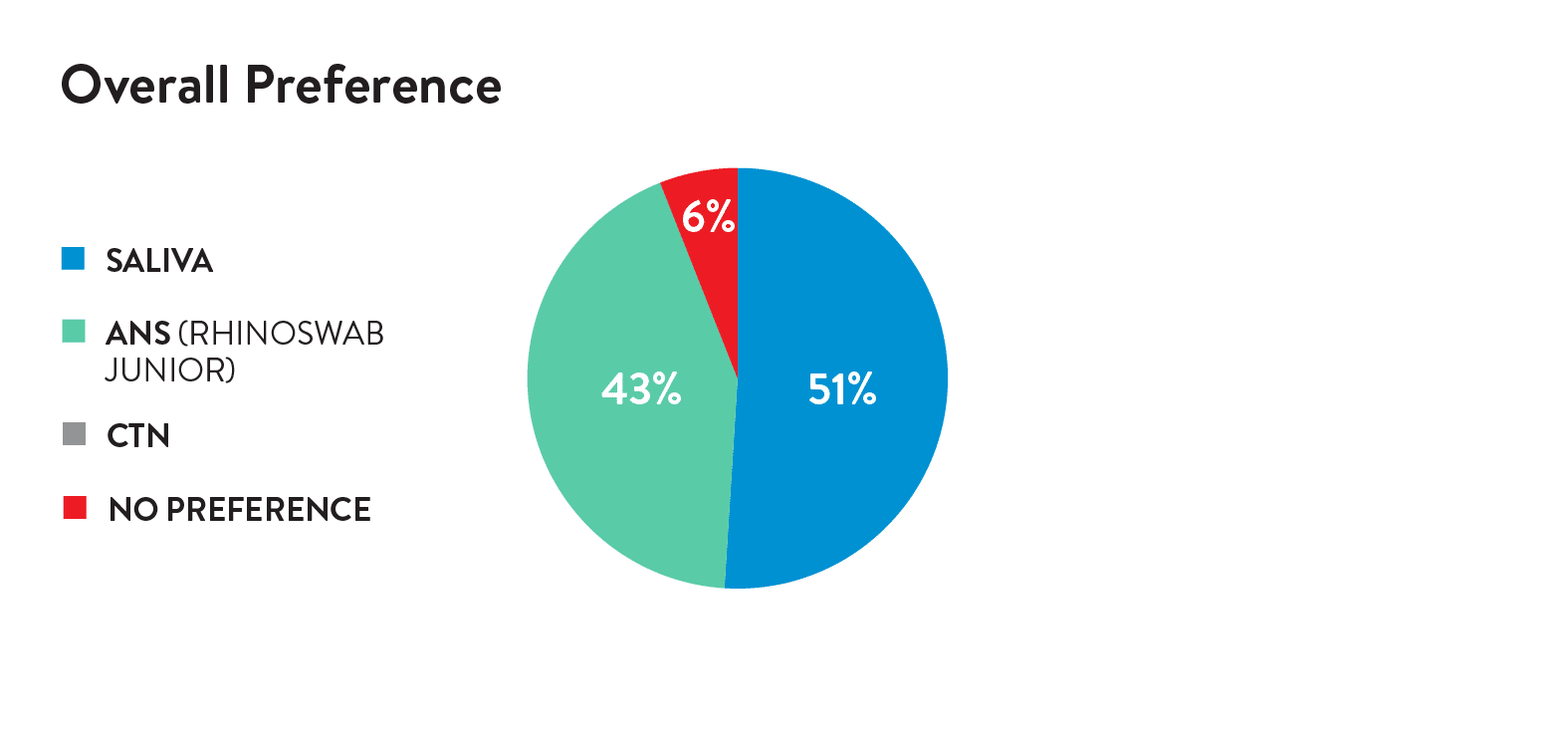
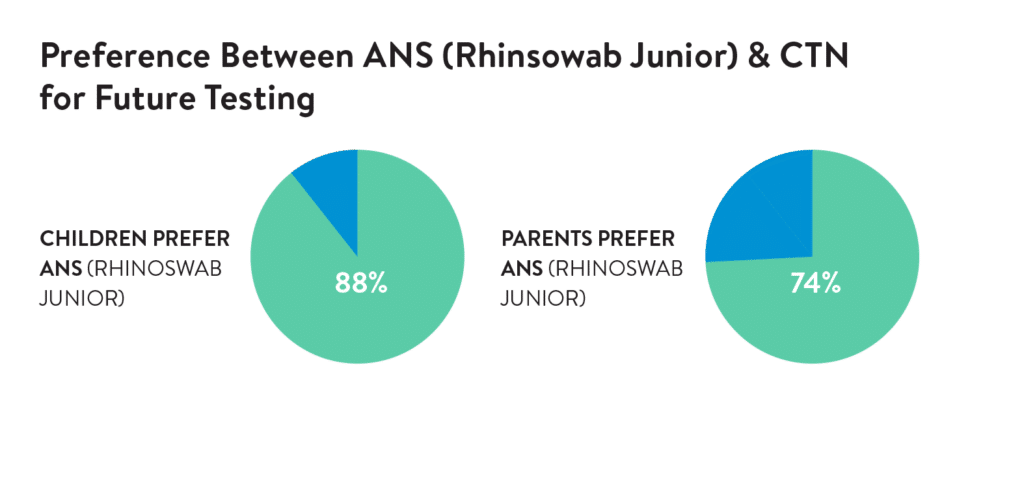
In a study of 53 children, 89.8% described Rhinsowab Junior as comfortable or a little uncomfortable, and 88% prefer Rhinoswab Junior over combined nose and throat swabs for future tests.
No. A usability study showed that 97.9% of participants (47 out of 48 participants who took part in the trial) reported that they experienced no pain at all when using the swab. Further, a user experience study conducted in 2021 by the Canisius Wilhelmina Hospital (CWZ) and the Radboud University Medical Center (Radboudumc) in the Netherlands, found that 98% of participants reported they experienced NO DISCOMFORT or PAIN when using Rhinoswab.
In a study of 53 children, 89.8% described Rhinsowab Junior as comfortable or a little uncomfortable, and 88% prefer Rhinoswab Junior over combined nose and throat swabs for future tests.
Yes, nasal swabbing is a valid sample collection method.
Swabbing only the nose is a valid sample collection approach. Scientific literature and guidance provided by the FDA, CDC (Center for Disease Control) and WHO support the efficacy and reliability of nasal swabs that access just the front of the nose rather than the depth of the nasal cavity.
No. Rhinoswab is not a test.
There are three stages to conducting a covid test; 1) Collect a sample 2) analyse the sample 3) report the results. Rhinoswab provides a comfortable alternative to standard nasopharyngeal swabs for collecting the sample. Once Rhinoswab collects a sample from the nose, the swab is placed in a standard vial containing saline transport media and sent to a lab for testing. The test is conducted within the pathology laboratory using rt-PCR testing equipment.
The Office of the Assistant Secretary of Health SARS – Co-V2 (COVID-19) Fact Sheet states: “Collection of nasal swab specimens is less technically complex, so can reduce the risk of the spread of infection to healthcare providers, by (1) reducing the duration of the procedure, and (2) allowing the patient to perform self-collection while under supervision.”
Self collection of nasal swab samples are safer for Health Care Workers who can supervise patients performing self collection from a distance rather than the HCW performing the collection.
The Office of the Assistant Secretary of Health SARS – Co-V2 (COVID-19) Fact Sheet recognizes that self collection of nasal samples “… also lessens PPE utilization, given that the patient can perform self-collection under supervision (versus the health care provider performing the collection).”
If you would like to trial Rhinoswab to determine how well it performs on your equipment or in your laboratory, please contact Peter Jordan, SVP Business Development, at pjordan@rhinomed.global to arrange a trial.
Rhinoswab Junior NEWS
- European Launch of Revolutionary New Swab for Testing Children for Respiratory DiseasesThe world’s most child-friendly respiratory swab was launched in Europe today at Medica 2022 in Dusseldorf, Germany. Rhinoswab Junior is the first nasal swab developed specifically for children. Its unique design addresses one of the pandemics’ … Read more
- New study says there is a better swab for testing children for covid and other virusesA new study suggests the traditional swab suffers from strong reluctance for use for children and a new Melbourne-designed Rhinoswab Junior is the preferred method of sample collection. The reluctance to test children for COVID-19 … Read more
- Study confirms new nasal swab is most accurate, comfortable and easy for kidsRhinomed is pleased to advise of the forthcoming publication of a further study and data by researchers from the Murdoch Children’s Research Institute and the Royal Children’s Hospital (RCH) Melbourne confirming the efficacy and comfort of the … Read more
- Surescreen Australia & Rhinomed – Rhinoswab Supply DealRhinomed Limited is pleased to report that it has finalised an exclusive supply agreement with SureScreen Australia to supply Rhinoswabs and Rhinoswab Juniors for inclusion in SureScreen’s class-leading range of lateral flow point of care … Read more
REquest a sample
Rhinoswab Junior is FDA, TGA and CE registered and available for trial or purchase. Rhinoswab Junior is currently available for professional and commercial use only and comes in a minimum order quantity of 1000 swabs.
If you would like to trial Rhinoswab Junior, please complete this form to request samples.
Or contact us to discuss how Rhinoswab Junior may help deliver a better sampling solution for your needs.