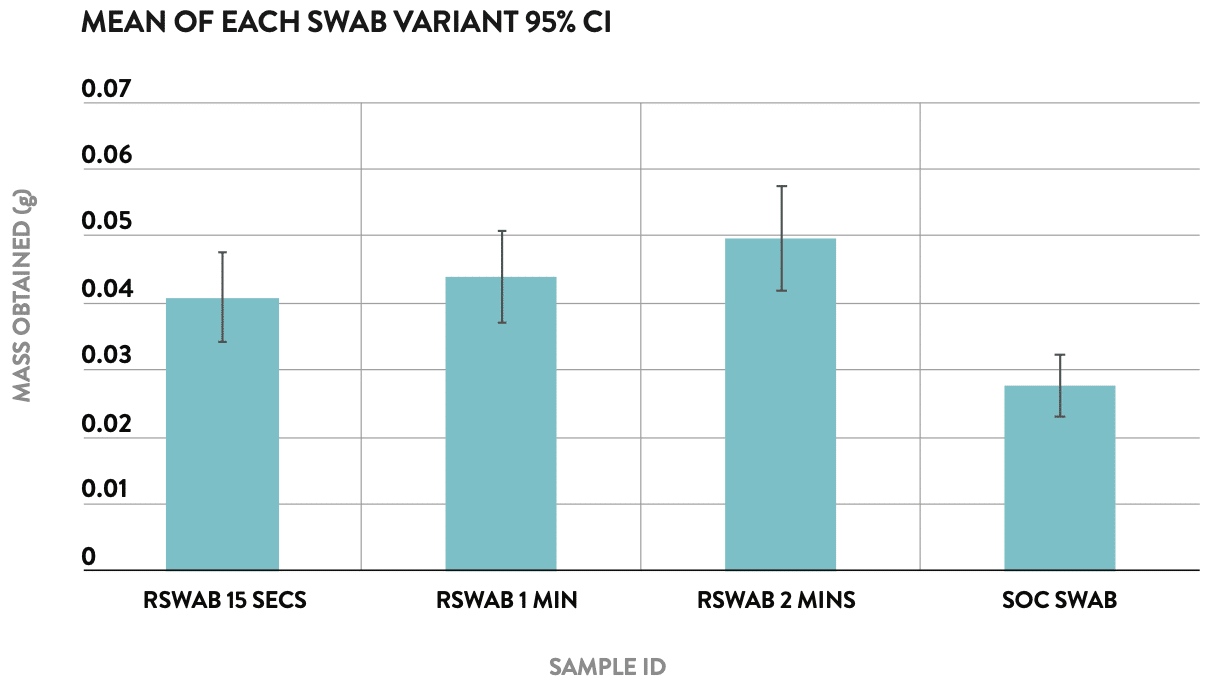
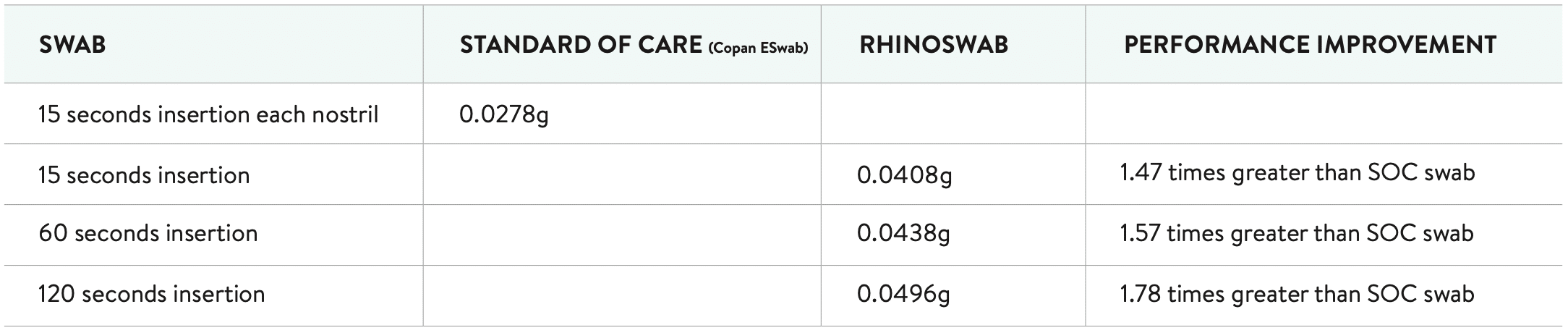
The objective of the nasal swab yield study was to compare the mean sample capture performance of the rhinoswab against the commercially available standard of care nasal swab (Copan ESwab™) at various insertion time points.
Design
An comparative experiment to measure the absorption profile of Rhinoswab when worn in the nose for different time periods (15 seconds, 1 minute and 2 minutes) versus the Copan ESwab™, used as per standard collection protocol of 15 seconds in each nostril.
Method
There were 50 study participants, all healthy volunteers of different ages and ethnic backgrounds. A total of 311 samples were self collected with the time of collection noted. The swab protocol was randomised to compare the Copan ESwab™ and Rhinoswab at 15 seconds, 1 minute and 2 minutes.
Swabs were measured before and after insertion to assess the sample mass collected. The sample mass obtained from each swab was determined by measuring the swab before and after nasal insertion using a calibrated Sartorius analytical scale (precision 0.0001g). The before and after weight was noted as well as the time of day that the sample was obtained.
Results
1. When compared to the Copan ESwab™ it was found that Rhinoswab collected a statistically significant (95% CI) greater average sample mass at every sample collection protocol (15 seconds, 1 minute, and 2 minutes).
The performance improvement offered by Rhinoswab in terms of sample capture is shown in the table below.
2. The time of day the sample was collected did not show any significant variation in the sample mass collected.